Ice Fishing
You will need
How to do it
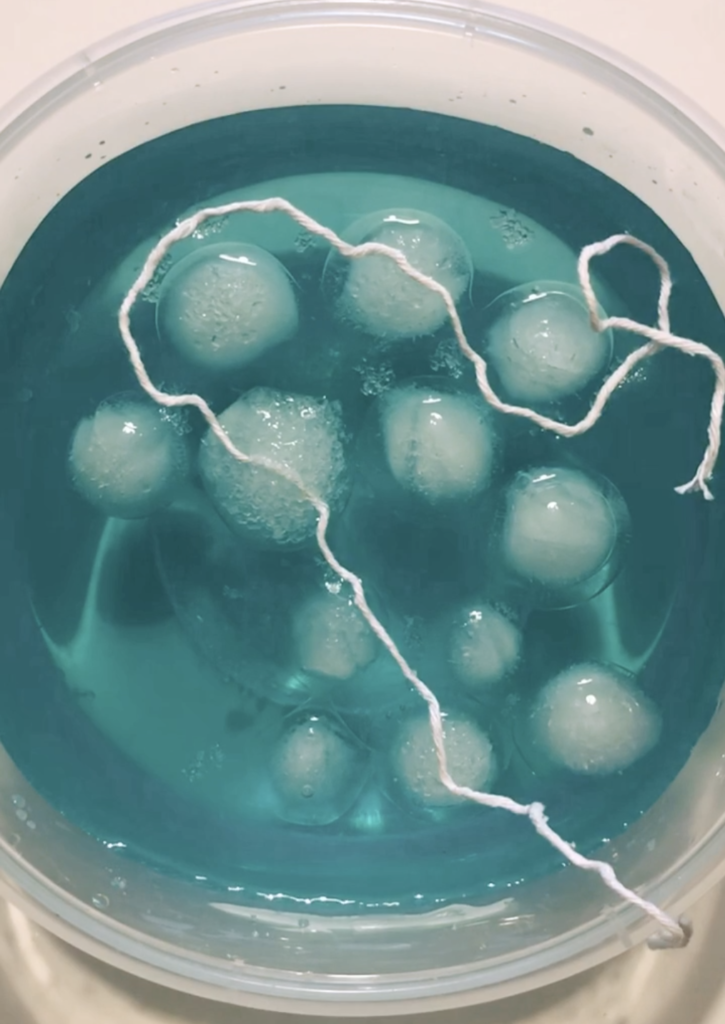
- Fill a bowl with cold water.
- Add a drop of food colouring to the water to create a colour contrast. Mix well.
- Drop in the ice cubes.
- Position your ‘fishing rod’ (string) across the ice.
- Sprinkle salt on the areas where the string and ice meet. Then leave for one minute.
- Lift up the string to see how much ice your fishing rod has caught!
What are we learning
Salt lowers the freezing point of water, helping to dissolve it faster. As it melts, the string sinks into the ice cube. The water dilutes the salt/water mixture, causing the ice on the top to refreeze, trapping the string.
Investigate
Find out about how salt is used on icy roads to keep them safe in the winter.